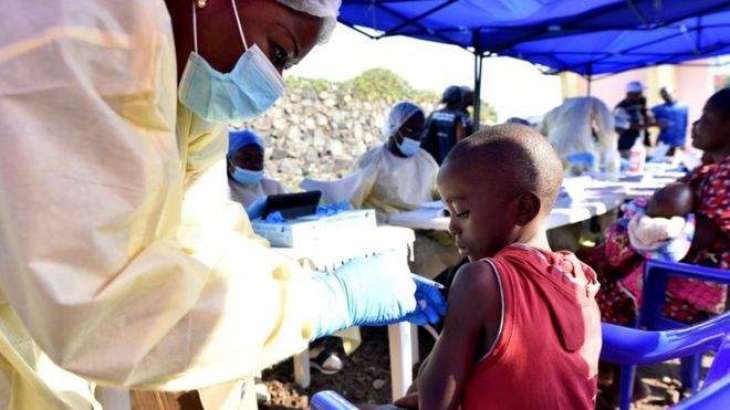
The European Medicines Agency (EMA) said on Friday it had recommended giving conditional marketing authorization for Ervebo, the first Ebola vaccine ever to be developed
MOSCOW (Pakistan Point News / Sputnik - 18th October, 2019) The European Medicines Agency (EMA) said on Friday it had recommended giving conditional marketing authorization for Ervebo, the first Ebola vaccine ever to be developed."EMA's human medicines committee (CHMP) has recommended granting a conditional marketing authorization in the European Union for Ervebo ... the first vaccine for active immunization of individuals aged 18 years and older at risk of infection with the Ebola virus," the EMA said in a statement.
EMA Executive Director Guido Rasi praised the development of the vaccine, calling it an important step in the fight against the disease.
"The CHMP's recommendation is the result of many years of collaborative global efforts to find and develop new medicines and vaccines against Ebola. Public health authorities in countries affected by Ebola need safe and efficacious medicines to be able to respond effectively to outbreaks and save lives," Rasi said, as quoted by the EMA.
Ervebo started being developed after the 2014-2016 Ebola outbreak in West Africa, according to the statement. The medicine was tested on some 16,000 people in Europe, Africa and the United States, and proved to be safe as well as cable of creating an immune system that is effective against the type of Ebola that spread in western Africa several years ago.
The Ebola virus is transmitted to humans from wild animals and is estimated by the World Health Organization to have a 50 percent fatality rate. The outbreak in West Africa, the worst one ever recorded, killed more than 11,000 people among the some 28,000 cases reported. The Democratic Republic of the Congo is currently facing such an outbreak.